Abstract

Background: Outcome for patients with ultra-high risk (UHiR) newly diagnosed multiple myeloma (NDMM) or primary plasma cell leukemia (pPCL) remains unsatisfactory. Recent results from MASTER (NCT03224507) and other trials unfortunately confirmed the sustained risk of post-ASCT relapse for UHiR MM patients in context of contemporary combination induction therapy, including relapse from undetectable minimal residual disease (MRD). The OPTIMUM/MUKnine (NCT03188172) trial was designed to reduce (pre- and) post-ASCT relapse risk in UHiR patients by continued Dara-VR(d) consolidation for 18 cycles, followed by Dara-R maintenance. We report here outcomes for OPTIMUM patients until end of consolidation therapy and contextualise results with outcomes of UHiR MM patients treated in the Myeloma XI (ISRCTN49407852) trial.
Methods: In OPTIMUM, patients identified to have UHiR NDMM (≥2 high risk lesions: t(4;14), t(14;16), t(14;20), gain(1q), del(1p), del(17p) or SKY92 risk signature) or with pPCL (circulating plasmablasts >20%) through central molecular screening of an all-comer population received up to 6 cycles of Dara-CVRd induction and V-ASCT. All patients then received 18 cycles of post-ASCT consolidation, of which 6 cycles Dara-VRd (Cons1) and 12 cycles Dara-VR (Cons2), before moving to monthly Dara-R maintenance until progression. OPTIMUM recruited 107 UHiR patients, including 9 pPCL, from 472 screened patients with suspected NDMM from 39 UK NHS hospitals between Sep 2017 and Jul 2019. We previously reported the OPTIMUM primary endpoint analysis, which demonstrated improved PFS at 18 months post start of induction for OPTIMUM in a pre-specified comparison against UHiR patients treated in the Myeloma XI (MyXI) trial. We report here a planned analysis of OPTIMUM patient PFS and safety data after all patients completed consolidation. Results are contextualised with outcome for UHiR patients treated in MyXI with carfilzomib/cyclophosphamide-Rd (KCRd) or CRd induction, ASCT and lenalidomide maintenance or observation. Further analyses including overall survival are underway and will be presented at the meeting.
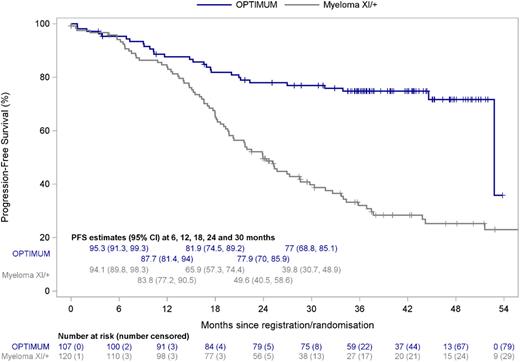
Results: With median follow-up of 40.0 months (95% CI: 37.3-42.7), median PFS was not reached for OPTIMUM patients. The PFS estimate at the end of consolidation, 30 months from start of induction therapy, was 77.0% (95% CI: 68.8-85.1). For context, PFS at 30 months for UHiR patients in Myeloma XI was 39.8% (95% CI: 30.7-48.9) for the overall group, and specifically 49.7% (95% CI: 33.2-66.2) for MyXI patients randomised to KCRd and 34.1% (95% CI: 23.4-44.8) for those randomised to CRd treatment arms (Figure 1). We observed a progressive separation of PFS curves over time between OPTIMUM and MyXI, suggesting a sustained beneficial effect of ongoing post-ASCT consolidation for UHiR patients (Figure 1). Of the 85 OPTIMUM patients who received at least one cycle of consolidation post-ASCT, 80 patients completed Cons1 and 74 patients Cons2. Progressive disease was the commonest reason for trial discontinuation. Most frequent CTCAE grade 3/4 adverse events (AEs) during Cons2 included neutropenia (43.8%), thrombocytopenia (27.5%) and infection (15%). Grade 4 events were rare (≤5%) for all categories; there were no treatment-related deaths. Peripheral neuropathy rate grade ≥2 was 13.8% (Grade ≥3: 2.5%) for Cons2; no cumulative increase from Cons1 was noted. Of the initial 107 patients who started therapy in OPTIMUM, 47 had an MRD test result available at end of Cons2 at time of abstract submission. Of these, 44 (93.6%; 41.1% of overall trial population) had undetectable MRD at 10-5 sensitivity by flow cytometry. In the majority of patients undetectable MRD was sustained since ASCT.
Conclusions: Prevention of relapse remains a key challenge in the treatment of UHiR MM/PCL patients, including for patients achieving undetectable MRD. OPTIMUM outcomes for extended Dara-VR consolidation in UHiR/PCL patients demonstrate 30 months PFS of 77% and compare favourably with MyXI, but also results from the MASTER trial, which recently reported 24-month PFS of 58% for UHiR patients defined by same criteria (JCO 2021). Safety data demonstrate manageable side effects with extended Dara-VR consolidation. Our results support extended, risk stratified post-ASCT therapy for UHiR MM/PCL patients.
Figure Legend: PFS Kaplan-Meier plot for UHiR/PCL patients treated in OPTIMUM and Myeloma XI.
Disclosures
Kaiser:Seattle Genetics: Consultancy; Pfizer: Consultancy; Karyopharm: Consultancy; Takeda: Honoraria; BMS/Celgene: Honoraria, Research Funding; GSK: Consultancy; Janssen: Honoraria, Research Funding; AbbVie: Consultancy. Hall:BMS/Celgene: Research Funding; Janssen: Research Funding. Bowles:AbbVie: Research Funding; Janssen: Research Funding. Garg:Janssen, Takeda, Navartis, Amgen, BMS, GSK: Consultancy, Honoraria; Janssen, Amgen, Takeda, Novartis: Consultancy, Other: Ad Board; Janssen, Amgen: Consultancy, Speakers Bureau. Jackson:BMS: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; J and |J: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria; GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptides: Consultancy. Cook:BMS/Celgene: Consultancy, Research Funding; Karyopharm: Consultancy; Sanofi: Consultancy; Takeda: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Amgen: Consultancy. Pratt:Janssen: Consultancy; Gilead: Consultancy; BMS/Celgene: Consultancy; Binding Site: Consultancy; Amgen: Consultancy; Takeda: Consultancy. Drayson:Abingdon Health: Current equity holder in private company. Owen:Astra-Zeneca: Honoraria; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Brown:Janssen: Research Funding; BMS/Celgene: Research Funding. Jenner:Janssen: Consultancy, Honoraria; Pfizer: Consultancy; GSK: Consultancy; Takeda: Consultancy; BMS/Celgene: Consultancy, Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal